pulselab@orange.ichtj.waw.pl
|
Pulse Radiolysis Laboratory pulselab@orange.ichtj.waw.pl |
|
Head: Prof. Krzysztof Bobrowski |
| Links | About | Members | Lectures/posters | Publications | Grants/Awards | Instruments |
Research at Pulse Radiolysis Group focuses on radiation effects in aqueous media, with particular emphasis on the identities and molecular structure of short-lived transients and reaction mechanisms in sulfur-containing compounds and porphyrins systems.
First topic involves application of radiation methods for the study of OH-induced oxidation of sulfur-containing alcohols, carboxylic acids, amino acids and their derivatives, and peptides. It is concerned with the characterization and quantification of reactive intermediates, such as hydroxysulfuranyl radicals, radicals and radical cations with various 2-center/3-electron bonds, reaction kinetics and the measurement of absolute rate constants for elementary processes involving these reactive intermediates, such as intramolecular proton and electron transfer, beta-fragmentation, decarboxylation, and reaction with molecular oxygen. The sulfur intermediates can undergo a variety of subsequent reactions depending on conformational flexibility of molecules and functional neighboring groups present in molecules. Part of these studies is done in collaboration with Professor K.-D. Asmus (Radiation Laboratory, USA) and Professor C. Schöneich (University of Kansas, USA).
Such features of radiation generation of intermediates can be useful in clarifying long-range damage in biological systems, such as oligopeptides and proteins with sulfur-containing residues. The major experimental methods applied are, in particular: pulse radiolysis with optical and EPR spectroscopic detection, and chromatographic methods (GC, HPIC and HPLC) for product analysis with high-resolution analytic equipment (DX500 Dionex). These studies are complemented by photochemical studies in collaboration with
Dr. G. L. Hug (Radiation Laboratory, USA) doing the the laser flash photolysis and Professor B. Marciniak (A. Mickiewicz University, Poznan)
doing steady-state photolysis.
The second major area of research involves investigation of redox reactions in porphyrins systems. The reactions were modified by changing central metals, axial ligands and substituents on porphyrins rings. The special concern was catalytic effect of porphyrins on CO2 reduction. Several rhodium and iron porphyrins were investigated, using radiochemical and photochemical methods in different solvents, shoving various catalytic activity.
The research shoved the crucial role of an intermediate, unstable low valence state of metal in the porphyrin center. Various oxidation and reduction states involving metal and porphyrin rings were also generated and observed in some particular classes of porphyrins and its homologues, tetrabenzoporphyrins and porphycenes. Depending on the metal in the molecules, the process concluded either in formation of 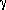 -radical ions of porphyrins rings or changing of the metals valence states. These research has been carried out at National Institute of Standards and Technology (USA) with collaboration with Dr P. Neta (NIST), Dr D. M. Guldi (Notre Dame Radiation Laboratory, USA).
-radical ions of porphyrins rings or changing of the metals valence states. These research has been carried out at National Institute of Standards and Technology (USA) with collaboration with Dr P. Neta (NIST), Dr D. M. Guldi (Notre Dame Radiation Laboratory, USA).