Research at Pulse Radiolysis Group focuses on oxidation effects in media, with particular emphasis on the identities and molecular structure of short-lived transients and reaction mechanisms.
 Subject involves application of radiation methods for the study of OH-induced oxidation of sulfur-containing alcohols, carboxylic acids,
amino acids and their derivatives, and peptides. It is concerned with the characterization and quantification of reactive intermediates, such as
hydroxysulfuranyl radicals, radicals and radical cations with various 2-center/3-electron bonds, reaction kinetics and the measurement
of absolute rate constants for elementary processes involving these reactive intermediates, such as intramolecular proton and electron transfer,
beta-fragmentation, decarboxylation, and reaction with molecular oxygen. The sulfur intermediates can undergo a variety of subsequent reactions depending
on conformational flexibility of molecules and functional neighboring groups present
in molecules.
Subject involves application of radiation methods for the study of OH-induced oxidation of sulfur-containing alcohols, carboxylic acids,
amino acids and their derivatives, and peptides. It is concerned with the characterization and quantification of reactive intermediates, such as
hydroxysulfuranyl radicals, radicals and radical cations with various 2-center/3-electron bonds, reaction kinetics and the measurement
of absolute rate constants for elementary processes involving these reactive intermediates, such as intramolecular proton and electron transfer,
beta-fragmentation, decarboxylation, and reaction with molecular oxygen. The sulfur intermediates can undergo a variety of subsequent reactions depending
on conformational flexibility of molecules and functional neighboring groups present
in molecules. 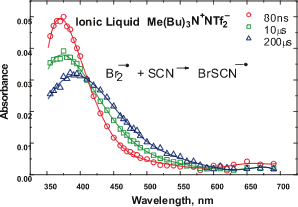 The second major area of research involves investigation of effects on some elementary chemical reactions in ionic liquids media.
Pulse radiolysis is a very effective method for a study of fast chemical reactions specially radicals and other transient species. The studies include redox reactions, reactions of dry and solvated electrons and some oxidizing species.
Room- temperature ionic liquids serve as good solvents for various thermal and electrochemical reactions and have been proposed as solvents for green processing. New kinds of ionic liquids are continuously developed and they are finding rapidly increasing numbers of applications.
The second major area of research involves investigation of effects on some elementary chemical reactions in ionic liquids media.
Pulse radiolysis is a very effective method for a study of fast chemical reactions specially radicals and other transient species. The studies include redox reactions, reactions of dry and solvated electrons and some oxidizing species.
Room- temperature ionic liquids serve as good solvents for various thermal and electrochemical reactions and have been proposed as solvents for green processing. New kinds of ionic liquids are continuously developed and they are finding rapidly increasing numbers of applications.